
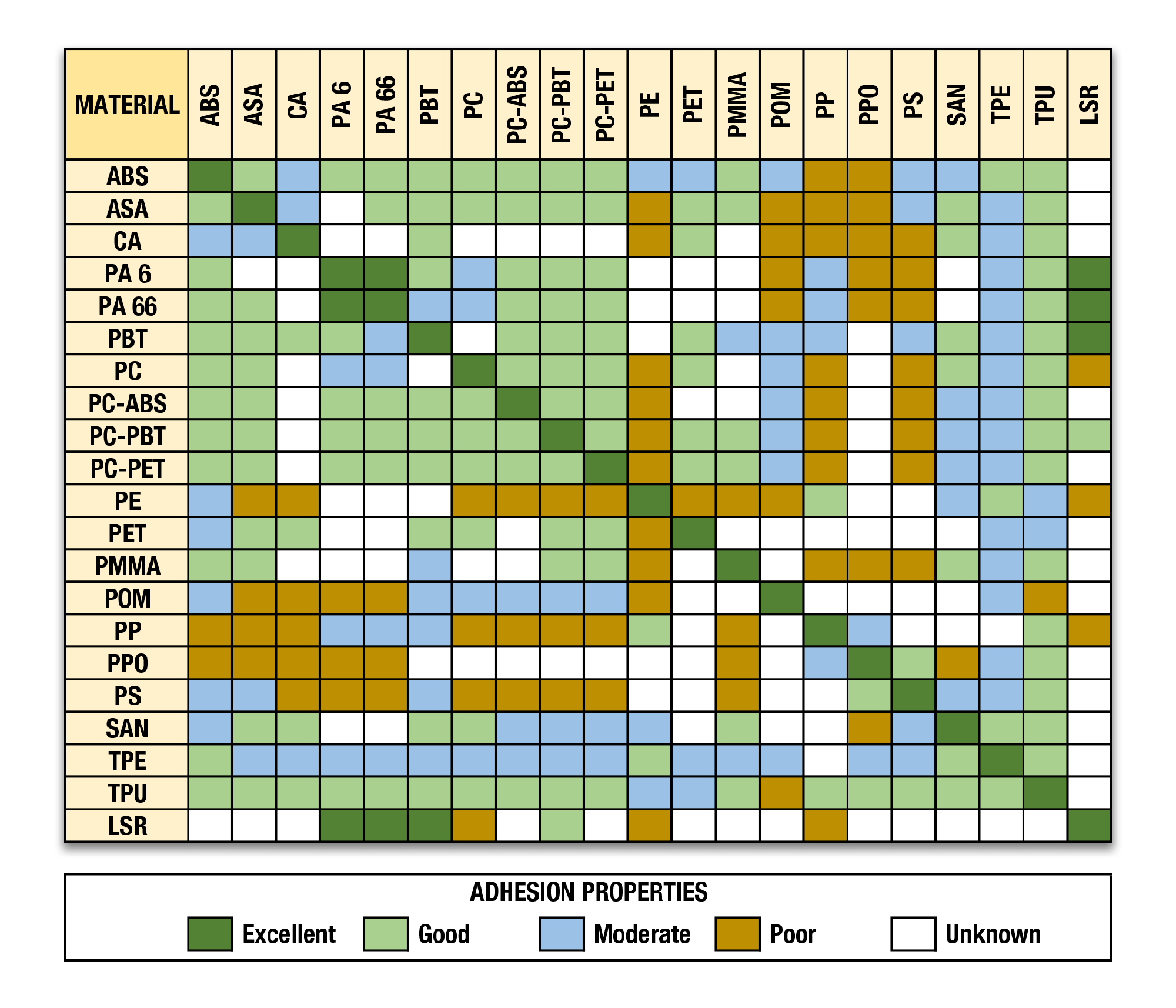
Ligand coupling method: aldehyde coupling via NH 2 residues of the ligandīinding capacity: 25–35 g IgG/L resin depending on flow rate, column height, and contact timeĮlution conditions: 20 mM citric acid or acetic acid, pH 3–4, or 20 mM sodium acetate, 1. Electronic musical instru- ment having data compatibility among different - class. Ligand: CaptureSelect™ FcXL affinity ligand Matrix: agarose-based, aldehyde-activated
CAPTO S CHEMICAL COMPATABILITY FREE
The VHH affinity ligand is a 12 to 15 kDa fragment comprising the three complementarity-determining regions (CDRs) that form the antigen-binding domain, efficiently produced in the yeast Saccharomyces cerevisiae by a production process free of any animal components (Animal Origin-Free). Consult the chemical manufacturer for best compatibility. Service conditions may vary and can affect accuracy of compatibility.
Capto S ImpAct is based on a high-flow agarose base matrix with good pressure/flow properties and an average bead size of 50 m, which combined with an optimized porosity gives high resolution. After the retentate was collected, a minimal volume of buffer was added for the UF rinse. Check chemical resistance with the connector supplier. During the UF/DF process, the antibody was initially concentrated to 90 g/L, diafiltered, and concentrated to ≥ 180 g/L, then the retentate was collected. Elution conditions: 20 mM citric acid or acetic acid, pH 34, or 20 mM sodium acetate, 1.0 M MgCl 2, 40 (v/v) propylene glycol, pH 56 Flow characteristics: 50200 cm/h (up to 2 bar) Formulation buffer: 20 (v/v) ethanol For Research Use Only. Before permanent installation, test the equipment with the chemicals and under the specific conditions of your application. Analysis showed that the Capto S run removed the excipients with yields of ≥ 96%. Material Clear Search WARNING The information in this chart has been supplied to Masterflex by other reputable sources and is to be used ONLY as a guide in selecting equipment for appropriate chemical compatibility. In addition, Capto S has lower resin costs, takes less time to process, and uses milder elution conditions. A Capto S column was chosen over Protein A chromatography to remove excipients from formulated drug substance because of its higher binding capacity.

of a resin whose chemical and economic compatibility with the food sector. Fortunately, a sufficient supply of formulated DS was available for reprocessing. leads to the denaturation of the protein (s) present in the liquid juice. Since the pilot plants were not available for large-scale campaigns, a creative alternative was needed to produce 2 kg of antibody from formulated DS for these studies. These larger molecules are collected in the. Capto S is available in a range of different bulk pack sizes and convenient pre-packed formats for easy scale-up and process development. The inactive shell excludes large molecules (average cut-offs: Mr 400 000 for Capto Core 400, and Mr 700 000 for Capto Core 700) from entering the core through the pores of the shell, see image below. Capto S withstands effective and rigorous CIP procedure and the chemical stability ensures long media lifetime. During process scale-up, the project team decided to make a high-concentration mAb drug substance for subcutaneous injection and change the formulation. Capto Core 400 and Capto Core 700 are composed of a ligand-activated core and an inactive shell. The scope of this work is two-fold: 1) excipients removal from formulated mAb drug substance by Capto S chromatography and 2) UF/DF process development to make high-concentration drug substance (DS) for subcutaneous injection.


 0 kommentar(er)
0 kommentar(er)
